Summary
Zur Historie der und zum naturwissenschaftlichen Urteil über die Wassergedächtnis-Hypothese
On the history and scientific judgment about the water memory hypothesis
A guest contribution by Dr. Carolin Sage
The “classic” among the attempts to declare a mechanism of action of homeopathic remedies. Also, occasionally popular elsewhere, e.g. with Edward Bach’s flower essences, where it is supposed to absorb and pass on the “cosmic vibrations” of its plants via sunlight (alternatively also by simply boiling them). If you think about it, the representatives of “spiritual medicinal power” suddenly become disdainful materialists when they pretend that water is imprinted with other material structures as an “imprint” in material structures, like a euro coin in a coin press (the solvent in potentizing is usually a water-alcohol solution, and lactose in trituration, so there should also be a lactose memory… well, let’s leave that aside, otherwise we’ll end up with globules at the very end of the chain of memories).
The hint that a permanent storage and a subsequent reading of information is impossible because of the liquid aggregate state of water can be found in several places on our websites. However, one can delve even deeper into the wondrous world of water, which makes the homeopathic hypothesis seem even more far-fetched.
We would like to thank Dr Carolin Sage, chemist and science journalist, for providing us with the following guest article. We would like to point to her informative website “Consider Science“.
The “water memory” from a chemical perspective
On the subject of “water memory”, a number of scientists have already achieved sad renown. In the 1960s, Fedyakin, Deryagin and the “polywater” caused a sensation: the Russian researchers conducted studies on water in thin glass capillaries and found that the properties of this water changed over time. The melting point was clearly lowered, while the boiling point and density were increased compared to conventional water. They were sure that the structure of the water itself had changed. However, the results could not be reliably reproduced by other researchers and a few years after the spectacular announcement of the polywater, the theory of a changed water structure was refuted by spectroscopic investigations by Lippincott and Stromberg.
The work of the French biomedical scientist Jacques Benveniste in the late 1980s was similar. He examined highly diluted solutions of antibodies. With his claim that water could form an immunological memory, Benveniste even made it into the scientific journal Nature. The attention of the research community was great and Benveniste had to face an investigating committee. Neither under the supervision of this committee nor by other scientists could the previously achieved results be reliably reproduced.
Another twenty years later, the French biophysicist Rey presented his work with lithium chloride dissolved in deuterated water – deuterium is one of the heavy isotopes of hydrogen – in extremely high dilutions. On the basis of thermoluminescence investigations, Rey seemed to have proved that signals of the lithium salt could be found even when there was no salt left at all. History repeated itself once again: the revolutionary work did not stand up to the scrutiny of the method and laboratory practice, the results were not scientifically tenable. This time, there was no outcry from the scientific community.
At about the same time, the American bioengineer Pollack again proposed a theory on water memory. Based on interactions on the surfaces of certain solid carrier materials, he defined a so-called exclusion zone in which the water molecules arrange themselves into a defined structure. In this structure, hexagonal layers are supposed to be stacked on top of each other, and the changed structure is supposed to be accompanied by changed properties such as density, charge, ability to store information, etc. The theory of the so-called hexagonal water has encouraged the pseudo-medical scene to speculate on all kinds of healing effects, always referring to serious scientific research. And indeed, numerous publications by Pollack can be found in quite serious scientific journals. But it is never about water memory; he publishes this “knowledge” in magazines like Edgescience or at symposia, e.g. at the German Society for Energy and Information Medicine.
So much for the historical overview. Let’s now look at water from a structural chemical perspective. What is it about the ability to form networks? What do such networks look like and how could information be stored in them?
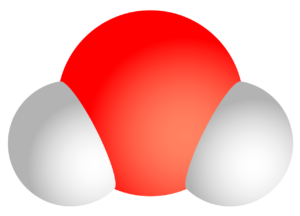
The water molecule H2O is a dipole. This means that the different electronegativity of the elements oxygen (O) and hydrogen (H) causes an uneven charge distribution to prevail in the water molecule. The oxygen atom is therefore able to attract further hydrogen atoms from other water molecules in addition to its “own” hydrogen atoms fixed in chemical bonds. This is a result of the different partial charges of oxygen (negative, since high electronegativity) and hydrogen (positive, since low electronegativity). This attractive interaction is called hydrogen bonding. It should be noted that the hydrogen bond, i.e. the attractive effect of one water molecule on another, is only about one tenth of a “real” bond.
The ability to form hydrogen bonds is linked to the idea that water can therefore form a defined structure within which dissolved substances would leave a kind of imprint. It is true that dissolved substances interact with water, provided they are polar, i.e. they themselves carry charge or have charge differences. This interaction also influences the local water structure. But (!): Hydrogen bonds have a very short lifetime. This is in the picosecond range. A picosecond (10-12 seconds) is the millionth part of a millionth of a second. So, water can actually be considered a network, but this fluctuates incredibly fast. A defined structure can therefore not be formed.
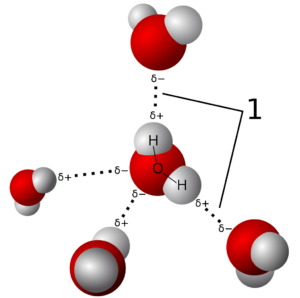
This is not possible even in partial areas. There is a lot of new work from water research that shows that it is possible to measure small clusters of about 5-6 water molecules, but they also only persist for a very short time. Quantum chemical computational methods support these measurements and make important contributions to estimating the energies of these particles.
Proponents of water memory often argue that in other storage media, such as a CD, no change in chemical composition is found by storing data. That is of course correct. But we are not talking about an elemental analysis, but a structural analysis. The properties of substances are, with a few exceptions such as flammability or similar, structure-dependent. This is easy to understand when you consider whether a melted, i.e. liquid, CD can still store data.
Conclusion: The possibility of storing information is centrally linked to a defined stable, non-fluctuating structure of atoms or molecules in a material. And such a structure does not exist in water.
We add: Even if such a structure were to exist, it would still be of little to no relevance to the homeopathic manufacturing process and for the medicinal relevance of homeopathy, A “water memory” or similar thoughts would have no explanatory value at all.
Literatur:
Rademacher, Die Mär vom Wasser mit Gedächtnis, Chem. Unserer Zeit, 47: 24-31, 2013.
K. Roth, H2O – Jo mei!, Chem. Unserer Zeit, 47: 108-121, 2013.
W. Mäntele, Elektrosmog und Ökoboom, Ein naturwissenschaftlicher Blick auf populäres Halbwissen, Springer Verlag, Berlin 2021.
G. H. Pollack: Wasser, viel mehr als H2O, VAK Verlags GmbH, Kirchzarten 2014.
R. Ludwig, Wasser: von Clustern in die Flüssigkeit, Angew. Chem., 113: 1856-1876, 2001.
R. Ludwig, D. Paschek, Wasser: Anomalien und Rätsel, Chem. Unserer Zeit, 39: 164-175, 2005.
Pictures:
Article image: public domain / own picture
Fig. 1: H2O (water molecule).jpg by Solkoll. Corrected by Plenz, public domain, https://commons.wikimedia.org/w/index.php?curid=2512664
Fig. 2: 3D model hydrogen bonds in water.jpg, public domain, https://commons.wikimedia.org/w/index.php?curid=14929959

